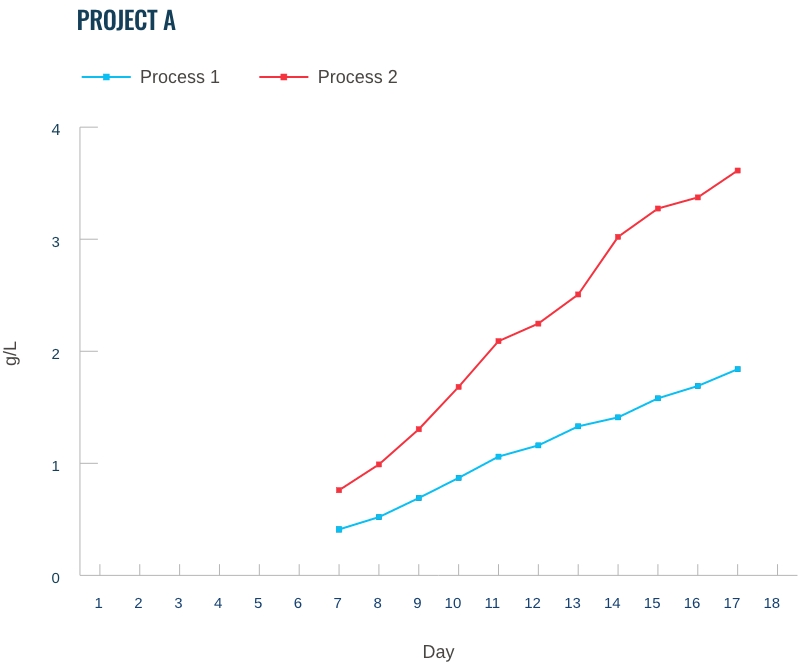
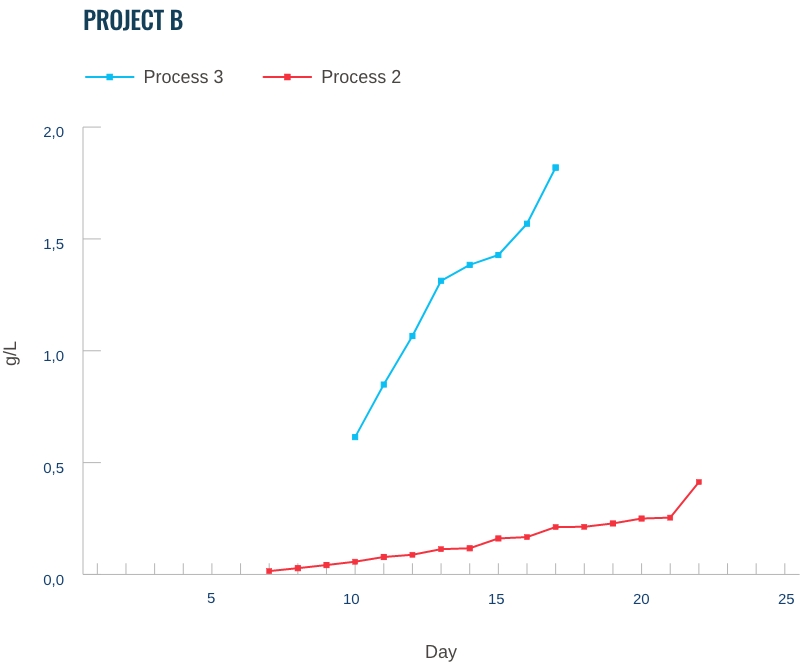
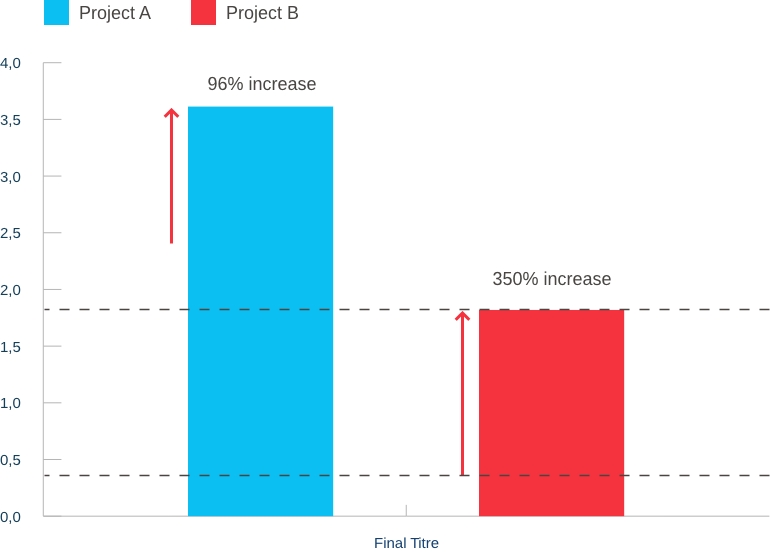
Our capabilities:
Process development
Culture media evaluation and optimisation
Cell line growth (duplication rate, cell metabolism and productivity) characterization and optimisation
Research Cell Bank Generation
Screening and identification of critical process parameters based on quality by design approach, with definition of process control strategies
Pre-Clinical material supply (Toxicology run)
Scale up to GMP scale
Scale down and process characterization
Harvest optimization to increase product yield and reduce impurities.